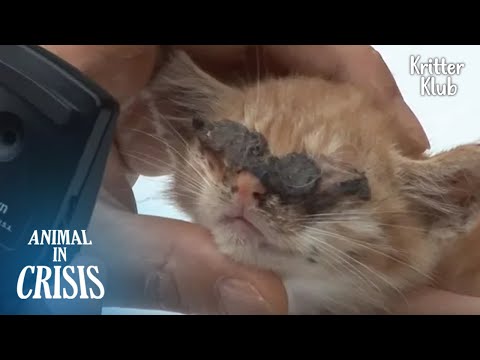
HVAC: Testing Refrigerant In An AC For Acid (QwikCheck QT2000) QwikCheck 2 Second Acid Test Kit
HVACR (Heating, Ventilation, Air Conditioning & Refrigeration) How To Check Refrigerant For Acid QwikCheck 2 Second Acid Test Kit (QwikCheck QT2000 Acid Test By Mainstream Engineering) Testing Refrigerant In An AC For Acid / Test Air Conditioner For Acid / Check Refrigerator For Acid
================================================
QwikCheck Detects inorganic acid in 2seconds down to 1 part per million in HVAC/R systems. Fast, accurate, and easy to use, QwikCheck provides an early warning of acid problems. The Yellow to Red color change allows you to easily and clearly communicate the need for action to the equipment owner. Works with all refrigerants and oils. Meets EPA venting regulations. For use, simply insert the valve core depressor into the center of the suctionside, vaporservice/Schrader valve while system is operating.
Qwikproducts #QT2000 By Mainstream Engineering
================================================
Refrigerant Acids and Their Treatment
We all know that the development of acids in the refrigerant of air conditioners, refrigerators or heat pumps can severely shorten the life of the compressor and the refrigerant. But how are these acids formed, and how fast can they burn out a compressor?
First let’s recall that acids are typically formed by chemical reactions with components and/or materials of construction, lubricating oils, and/or impurities. Elevated temperatures can accelerate the instability of the refrigerant and cause the formation of acids. This may be the result of improper operation, such as a failed condenser fan or a clogged airflow path. Checking for acid is a common maintenance recommendation since acidic conditions can be easily cleaned up before a compressor motor burns out. After a burnout, proper clean up is much more difficult.
If a compressor does burn out, the oil becomes extremely acidic. If all of the acid is not removed, the elevated acid levels will attack the new compressor and cause a premature motor burn out. Acid cleanup after a burn out must include changing the compressor oil and the refrigerant to reduce the acid level (and changing the compressor) as well as installing a new filterdrier. Unfortunately, however, changing the refrigerant and oil in a system still leaves trace amounts of the existing highly acidic oil
throughout the system. Research indicates that residual acid shortens the life of any new compressor because the residual acid will further accelerate acid formation in the system. A recommended procedure is to add a suction line filterdrier to trap any acid lodged in the system
before it can flow back to the compressor. The purpose of this filterdrier is to keep the return flow to the compressor as acidfree as possible, therefore, the filter should be located as close to the compressor suction as practical (since these suction lines could also be contaminated with residual acid). Even with new refrigerant, oil, and liquidline filterdrier supplemented with a suction line filterdrier, there still must be some method of removing or flushing the residual acid from the system (note that I said removing or flushing, and not neutralizing the acid). Some illinformed technicians often make the costly error of believing that the addition of a foreign substance (which forms a chemical reaction to
neutralize the acid) will solve their acid problems. This is not the case.
Remember that every neutralization reaction must follow the basic laws of chemistryacid and base react to form salt and water. The salt that forms is a corrosive metallic salt, which remains in the system to corrode components and potentially clog small passageways (such as bearing oil feed lines and expansion devices). As we know, every acid neutralization reaction leaves a residue. However, some manufactures have created a formulation where the solid residue is dissolved in another foreign substance to form a liquid residue (instead of a solid residue).
This is not a better situation. Adding more foreign substances to dissolve the salt into a corrosive liquid form makes it even more mobile and therefore potentially more damaging. In addition, the solids can
still precipitate to clog small passages. Why would anyone intentionally add solvents into a system to make the salt more soluble, and thereby making the potential problem worse? The answer is marketing
(not engineering)….this method allows manufacturers to deceptively claim it “leaves no SOLID residue.” If you think that is bad, well some manufacturers have even gone so far as to claim that an acidbase
neutralization reaction leaves no residue. How is this possible, since every chemical reaction will form products of the reaction? Simple…. take a lesson from politics, and alter your definition of residue!
================================================
Thanks for watching!
Email: [email protected]
Follow me on Instagram! @JumpRmantech