CIDP and Efgartigimod
Richard Lewis, MD, Department of Neurology, CedarsSinai Medical Center, explains what CIDP is as well as the results of the recent AANEM meeting.
Transcription:
My name is Richard Lewis. I'm a Professor of Neurology at CedarSinai Medical Center in Los Angeles. I've been the past President of the Peripheral Nerve Society and Chair of the Inflammatory Neuropathy Consortium.
One of my interests has been in inflammatory neuropathies. The most common acute inflammatory neuropathy is called Guillain–Barré syndrome, which you may have heard of. That's a monophasic illness where people from the time they start having symptoms to the time they're at their worst is about three weeks, sometimes four, and they can be completely paralyzed. But fortunately, with some treatments and with time, most of those people get significantly better and can ambulate.
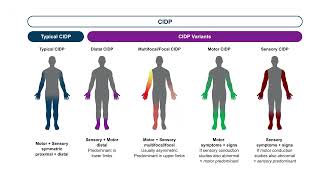
The disorder we're going to talk about today is CIDP or Chronic Inflammatory, Demyelinating Polyneuropathy. It has a lot of relationships to Guillain–Barré syndrome. The difference being that its progression is greater than eight weeks. Now it could be relapsing or progressing, but the disorder continues to progress and requires ongoing treatments.
There are treatments for it. There are three that are considered the primary treatments, which are corticosteroids, intravenous immunoglobulins, IVIG, or its companion subcutaneous immunoglobulin, and plasma exchange. There are other treatments that may work, but we don't have the evidence that proves that they work, but they're all immunosuppressants of various types.
There has been a need to find new treatments that might be able to stop, halt, and control the disease in ways that the other treatments can't, because no treatment is successful in every patient, whether it be that they're not effective in some patients or the patient has side effects. But we need new treatments.
Most recently, Argenx has announced that there's a compound that they're looking at, which is called efgartigimod, which is an FcRn inhibitor. A lot of letters. What it basically does is reduce the amount of circulating IgG or Immunoglobulin G. When you get IVIG, they give you 10,000 people's IgG, and those IgG block the pathogenic things that are going on in CIDP and also in Guillain–Barré syndrome. It's one of the main treatments of Guillain–Barré syndrome.
FcRn inhibitors do the exact opposite. They knock down your IgG, which we weren't sure at the time, but we thought was a major player in what causes CIDP. Unfortunately, we don't really know the cause of CIDP, which is actually a syndrome. There are a number of different presentations, and the likelihood is that there's not one specific cause, but many.
This trial looked at a subcutaneous form of efgartigimod, where patients get it weekly. The injection only takes about 90 seconds, so it's quick, pretty easy to do. They basically took patients with CIDP and then had them go off their prior treatments, which most of the patients were IVIG. When they relapsed, they treated them with this medication.
What they found was that over twothirds of the patients who had gotten worse when they went off their IgG got better with this treatment. Now, twothirds doesn't sound like a lot, but when you look at it, it was a little closer to about threequarters of the patient, and it's quite a striking response to the treatment. One of the things this trial did that no other trial had done before was to really delve deep to make sure that the patients had CIDP.
Then the trial took all the patients who had responded to that initial treatment and then randomized them to getting the medicine or not getting the medicine, getting placebo. What they showed is that within eight weeks, the majority of placebo patients had relapsed, whereas the majority of patients with the efgartigimod did not relapse. There was a significant protective effect of staying on the efgartigimod that lasted up to a year.
The conclusion that we reached, and we've looked at it a lot of different ways, is that one, there is clearly an effect of this medicine on the majority of patients, that this means that IgG, immunoglobulin G, the certain form of immunoglobulin is particularly important in the cause of the disease in these patients, that being on it will keep you sustained with improvement for up to a year. We don't know any longer because the trial did not go any further than that. It's a very exciting trial and is the first new treatment for CIDP in over 30 years. We're very excited about this, and it gives a new possibility to treat this disease, which in many ways is going to be an advantage over some of the other medications that we currently have.
To learn more: https://checkrare.com/diseases/neurol...
https://www.aanem.org/