27.1 Introduction to Nuclear Physics | General Physics
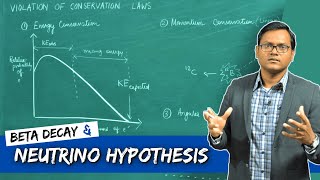
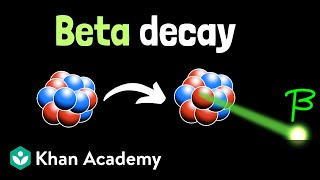
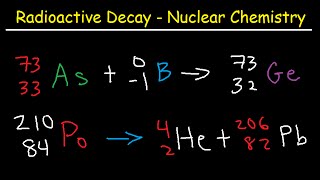
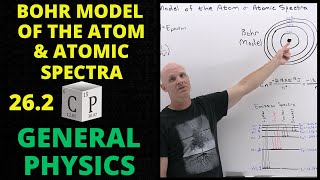
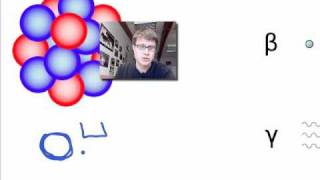
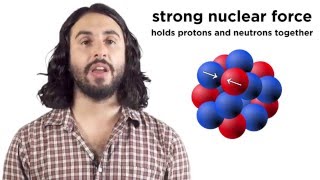
Chad provides an Introduction to Nuclear Physics. The lesson begins with an introduction to a variety of nuclear particles: alpha particles, neutrons, protons, beta particles (electrons and positrons), and gamma rays. Characteristics for each are provided including mass and charge. Atomic symbols are then reviewed, showing how the composition of a nucleus (number of protons and neutrons) can be determined from it, which is related to the stability of that nucleus. The Belt of Stability is presented showing that stable nuclei fall within a narrow range for the ratio of neutrons to protons. For the lightest nuclei, a 1:1 ratio is optimal, but as nuclei increase in mass, a slight excess of neutrons is optimal. This is the result of a balance of the attraction of the strong nuclear force between all the nucleons (protons and neutrons) and the electrostatic repulsion between the protons. The overall balance of these forces is directly related to the stability of the nucleus, which is inversely related to the likelihood of the nucleus being radioactive. Increased stability results in a decreased likelihood of radioactivity, and a decreased stability results in an increased likelihood of radioactivity.
The potential energy associated with the strong nuclear force is referred to as the Nuclear Binding Energy. Effectively, the nuclear binding energy is defined as the energy required to break apart a nucleus into its constituent nucleons. It turns out that the mass of a nucleus is always less than its constituent nucleons. It is that difference in mass, termed the mass defect, that is converted into energy (E=mc^2) which serves as this nuclear binding energy.
00:00 Lesson Introduction
00:50 Nuclear Particles
07:39 Nuclear Binding Energy
Check out Chad's General Physics Master Course: https://courses.chadsprep.com/courses...




![Alpha, Beta & Gamma Decay [Complete Discussion]](https://i.ytimg.com/vi/eUEgpcQHzIA/mqdefault.jpg)