26.2 Borhr Model of the Atom and Atomic Spectra | Quantum Physics
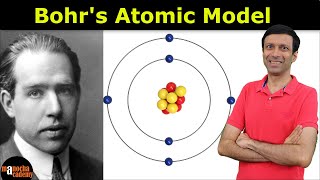
Chad provides a thorough lesson on the Bohr Model of the Atom and Atomic Spectra (line spectra) related to it. In 1905 Niels Bohr proposed a quantized model for the hydrogen atom the equations derived from which matched the absorption and emission spectra for hydrogen. The first tenet of his model was that electrons move around the nucleus in circular orbits. The second tenet is that only certain energies and therefore radii for these orbits were possible. The third tenet was that photons of light are absorbed or emitted when electrons change orbits, and that the energy of the photon was equal to the difference in the energy of the orbits. Bohr's model predicted the wavelengths of all the photons observed for the absorption and emission spectra for hydrogen, but failed for any atoms having more than 1 electron. His model was not completely correct, but it did correctly state that the energies of electrons in atoms are quantized.
The formula for electron energies in the hydrogen atom is provided as well as the formulas for calculating the energy and wavelength of absorbed and emitted photons corresponding to the line spectra for hydrogen (including the Rydberg equation). Four common emission spectra series are presented: Lyman, Balmer, Paschen, and Brackett with particular emphasis placed on the Balmer series as many of the emission lines in the Balmer series are in the visible region of the spectrum. Sample calculations for each are performed.
00:00 Lesson Introduction
00:59 Bohr Model of the Atom
05:14 Atomic Spectra (Absorption and Emission)
09:49 Atomic Spectra Calculations
Check out Chad's General Physics Master Course: https://courses.chadsprep.com/courses...