Mole Concept Class 11 | Chemistry
This lecture is about mole concept class 11. In this animated lecture, I will teach you the super easy concept of molar mass, relative mass and mole concept in one shot. Also, you will learn the mole calculation without using formula.
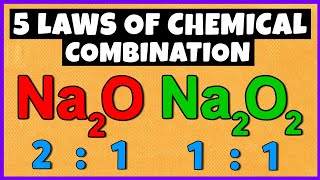
Q: What is the easy concept of mole?
Ans: The easy concept of mole in chemistry is that it is equal to the molar mass of any element.
For example,
1 mole of carbon atom = 12 grams
1 mole of oxygen atom = 16 grams
1 mole of nitrogen atom = 14 grams
Secondly, in 1 mole of any substance, there are 6.023 into 10 to the power 23 atoms or ions or molecules are present. This number is commonly known as Avogadro's number.
To learn more about mole concept, watch this lecture till the end.
#moleconcept
#numericals
#chemistry
Subscribe my channel at: / @najamacademy
Youtube link: / @najamacademy
Facebook link: / najamacademy