17.5 Common Ion Effect and Precipitation | General Chemistry
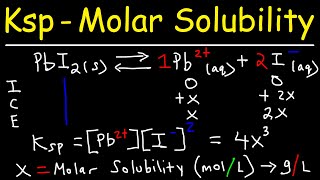
Chad continues with a second lesson on solubility equilibria covering the Common Ion Effect and Precipitation. The solubility of a salt is decreased when a strong electrolyte having a common ion is present in the solution according to Le Chatelier's Principle. Chad explains how to account for this mathematically using an ICE table, showing precisely how these calculations are different than the solubility calculations covered in the last lesson.
A discussion of Qsp vs Ksp is then used to explain the process of precipitation of a salt from solution. If the ion concentrations in the solution are sufficiently high that Qsp is greater than Ksp, then a precipitate will form according to Le Chatelier's Principle. If the ion concentrations are sufficiently low that Qsp is less than Ksp, then the solution is unsaturated. And if the concentrations result in Qsp = Ksp, then the solution is saturated (but no precipitate will form). Example calculations are performed to demonstrate these principles.
I've embedded this playlist as a course on my website with all the lessons organized by chapter in a collapsible menu and much of the content from the study guide included on the page. Check this lesson out at https://www.chadsprep.com/chadsgener...
If you want all my study guides, quizzes, final exam reviews, and practice exams, check out my General Chemistry Master Course (free trial available) at https://www.chadsprep.com/genchemyou...
00:00 Lesson Introduction
00:25 Common Ion Effect
01:22 Calculating Molar Solubility with Common Ion Effect #1
06:09 Calculating Molar Solubility with Common Ion Effect #2
10:50 Introduction to Precipitation
18:26 Qsp vs Ksp: Does a Precipitate Form?
https://www.chadsprep.com/
https://courses.chadsprep.com/pages/p...